How Might Emission Spectra Be Used in Studying Stars
An instrument for obtaining a spectrum. Hint- think about how many energy levels are in each element 6.

Atomic Emission Spectrum Easy Science Electron Configuration Electromagnetic Spectrum Easy Science
It lets you see the chemicals being absorbed or emitted by the light source.
. It is the ultimate form of remote sensing. How might emission spectra be used in studying stars. The science of spectroscopy is quite sophisticated.
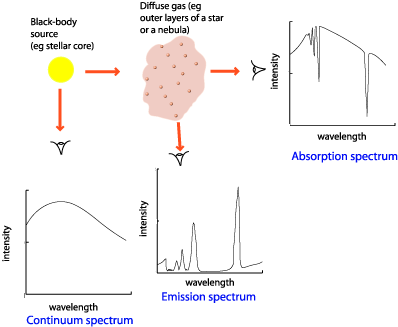
Solar spectrum showing the dark absorption lines. The spectral line also can. Absorption present are created when light from something hot like a star passes with a cooler gas cancelling out the emission lines the chemistry in the gas would typically create.
Webew7 and 7 more users found this answer helpful. A series or pattern of bright lines superimposed on a continuous spectrum. One can also compare which stars are the oldest and youngest based on the brightness of a star.
Weve been talking about the color of visible light out of convenience but this all applies to the invisible parts of the spectrum beyond the visible too. An emission spectrum occurs when the atoms and molecules in a hot gas emit light at certain wavelengths causing bright lines to appear in a spectrum. How many electrons can one orbital hold.
Spectra can be used to reveal the physical nature of stars. This tells us what type of. Today this process uses instruments with a.
In astronomy usually attached to a telescope to record the spectrum of a star galaxy or other astronomical object. You take the light from a star planet or galaxy and pass it through a spectroscope which is a bit like a prism letting you split the light into its component colours. So elements can be identified by the colors their atoms produce when energy by heating or electric current is used to reveal their emission fingerprints.
How might emission spectra can be used in studying stars. Absorption and Emission Spectra. The most common method astronomers use to determine the composition of stars planets and other objects is spectroscopy.
How to identify the Emission Spectrum. As with absorption spectra the pattern of these lines is unique for each element. Measuring the spectrum of light from a star can tell astronomers what the star is made of.
Imagine observing the light being emitted from a very hot star. How might emission spectra be used in studying stars. You can use spectra to estimate the temperature of the star.
One use of this technique is to identify the elements present in distant stars. From this it is possible to see the stars composition and age. When you look in the spectrum of a star because that example you have the right to see absorption lines due to the fact that the stars outer environment is cooler than the main part.
We can see emission spectra from comets nebula and certain types of stars. A graphic representation of Weins Law. A spectrum is a record or a graph of the amount and wavelength of light given off by any light source.
The light from the stuff between the stars allows astronomers to study the interstellar medium ISM. That energy holds the valence electrons in. The spectrum may be of.
Why do larger gases such as Neon produce more color bands line spectra than smaller gases like Hydrogen. The color of the stars can determine what major elements are on the star each emission spectrum is unique to specific element. How are emission spectra used in studying stars.
The different colors of light produced by emission spectra of different elements allows them to be identified. This type of study is called spectroscopy. Describe how is emission spectra produced.
3Identify unknown metals ions based on the results of the flame test. This material may be falling onto the star from a doughnut-shaped disk around the star called an accretion disk. Bright Line Spectra Write the name of each element located in Part I.
2Use a flame test to observe the color produced when metal ions are heated. The emission spectra are unique to each element because all atoms of one element are identical and each atom has a specific number of valence electrons with a specific level of energy present. Since each element emits or absorbs light only at particular wavelengths astronomers can identify what elements are in the stars from the lines in their spectra.
Most of the Suns energy is concentrated towards yellow. Emission spectra may be used in studying stars in order to determine what atoms makeup the individual star produces due to the fact that each atoms emission spectra biunique one can observe the spectra emitted by the star and identify the atoms that are released by the light the star produces. These disks often form around a neutron star or black hole.
Astronomers may use emission spectra in order to classify the atoms or ions from stars or the sun that send out light. Thus astronomers can identify what kinds of stuff are in stars from the lines they find in the stars spectrum. Each star doesnt emit the same proportion of energy in each color.
If you can stare at this light unhindered with no dust and gas between you and the object you may be lucky enough to observe a perfect blackbody a. Emission spectra are often referred to as elemental fingerprints. We can diagnose what they are made of and how they shine purely by.
Identifying elements in astronomical objects using their spectra. We do not have to visit the stars or bring back stellar material into the lab. 1Observe the bright line spectra emission spectra for various elements.
Into data table 1 located below. From this you can work out all sorts of things says Watson. Spectral information can also tell us about material around stars.
Emission spectra may be used in studying stars in order to determine what atoms make up the individual star produces due to the fact that each atoms emission spectra is unique one can observe the spectra emitted by the star and identify the.

Why Is Absorption Spectroscopy Preferred Over Emission Spectroscopy Quora
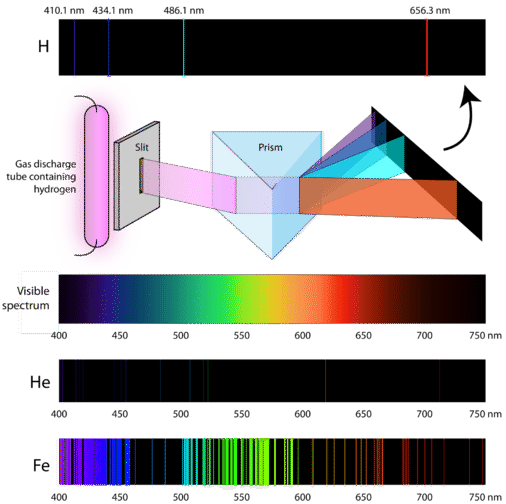
4 2 Understanding Atomic Spectra Chemistry Libretexts
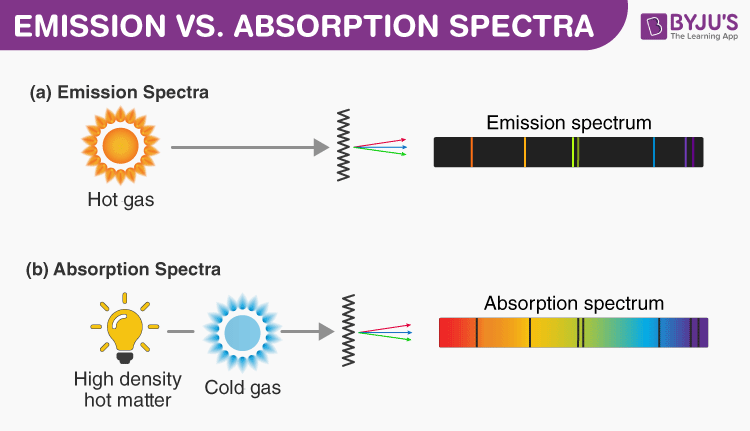
Difference Between Emission And Absorption Spectra Comparison Chart

This Was On My Chemistry Worksheet Pretty Sure I Can T Click On The Link With My Pen Chemistry Worksheets Chemistry Cards Against Humanity
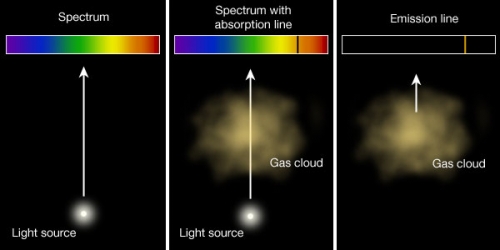
Kirchoff S Laws And Spectroscopy
Why Is The Emission Spectrum For Hydrogen Described By The Balmer Series Instead Of The Lyman Series It Has Only One Electron Orbital So Shouldn T Its Lowest Energy State Correspond To N 1
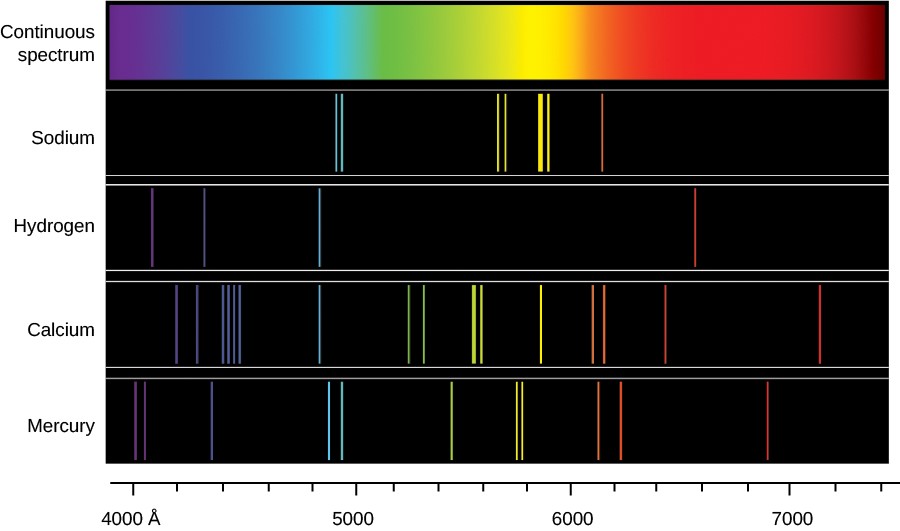
Spectroscopy In Astronomy Astronomy

Using Emission Spectra To Determine What Stars Are Made Of Youtube
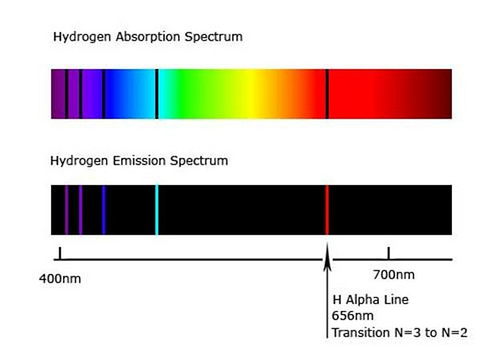
Absorption Spectrum Emission Spectrum Lines Article Khan Academy

Absorption And Emission Spectra Physics Notes A Level Physics Notes Physics
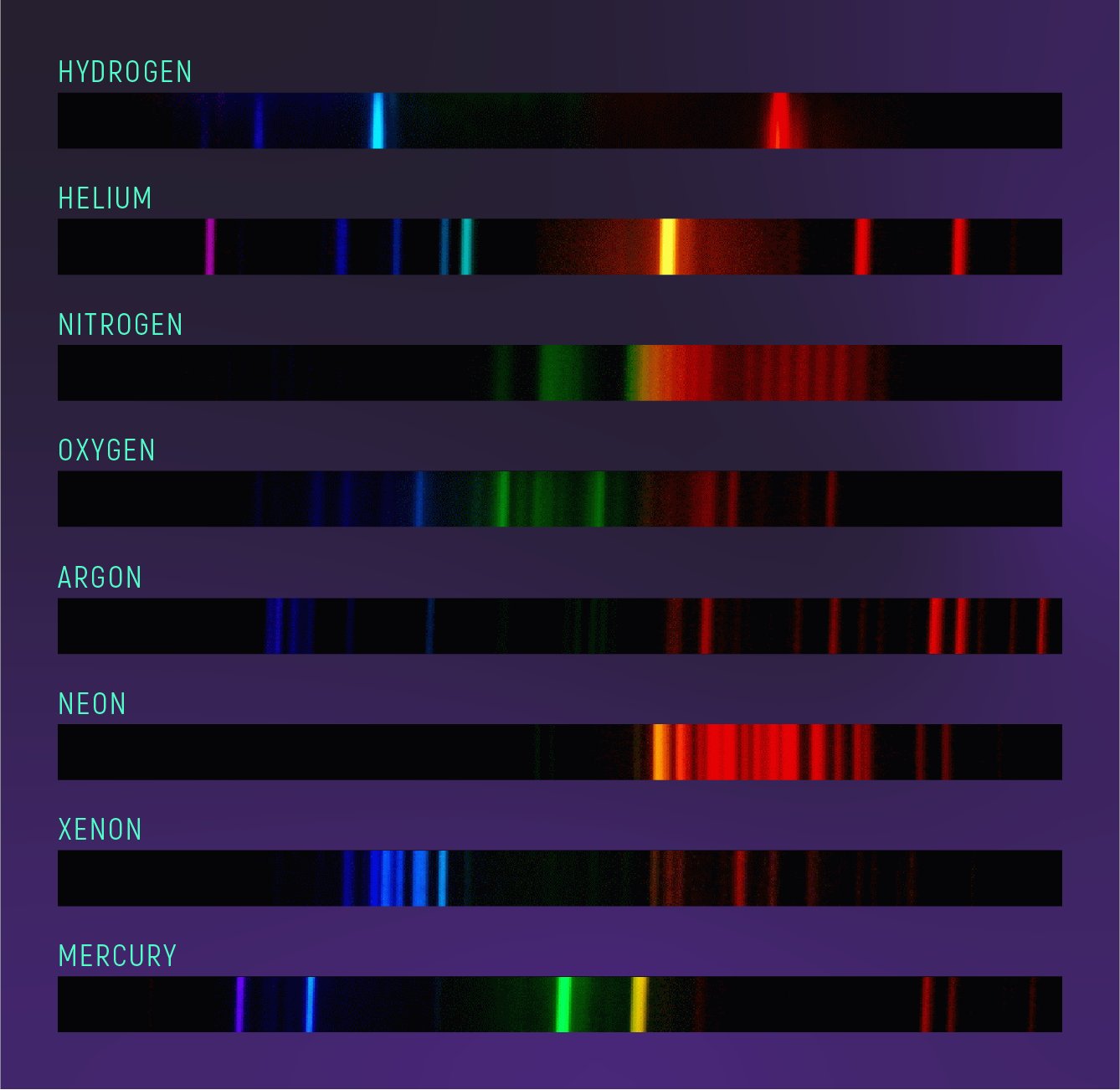
Space Telescope Science Institute On Twitter A Spectrum That Is Mostly Dark With Bright Lines Of Color Is Known As An Emission Spectrum It Is The Inverse Of An Absorption Spectrum Emission

Astronomy 1600 Guelph Week 6 Unit 6 Light Spectra Flashcards Quizlet
Comments
Post a Comment